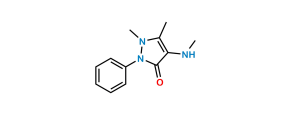
Metamizole Related Products
Vsquare is a leading innovator in pharmaceutical reference standards. We supply high-quality Reference Standards of Metamizole, pharmacopeial and non-pharmacopeial Metamizole impurities, metabolites, stable isotope products, and nitrosamines (N-NO products). Metamizole impurities reference standards are useful in pharmaceutical research. They are useful in product development, ANDA and DMF filing, quality control (QC), method validation, and stability studies. It is also useful in the identification of unknown impurities and the assessment of genotoxic potential.
Metamizole-related products are thoroughly characterized. Metamizole products are supplied with detailed COA & analytical data meeting regulatory compliance. We can also provide EP/USP traceable standards based on your requirements. The supplied products are re-tested at regular intervals.
Pharmaceutical Reference Standards
Possible Nitroso Standards
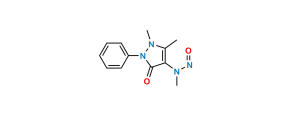
N-Nitroso Metamizole EP Impurity C
| CAT No | : VV-M001003 |
| CAS No | : 73829-38-6 |
| Mol.F. | : C12H14N4O2 |
| Mol.Wt. | : 246.3 |